Chapter 3 Metal and Non Metal (End Questions)
- Which of the following pairs will give displacement reactions?
(a) NaCl solution and copper metal
(b) MgCl2 solution and aluminium metal
(c) FeSO4 solution and silver metal
(d) AgNO3 solution and copper metal.
Ans: option d) AgNO3 solution and Copper metal will give displacement reaction.
Explanation: write the reactive series, here upper element can replace lower element, but lower element cannot replace upper element.
K
Na
Ca
Mg
Al
Zn
Fe
Sn
Pb
H
Cu
Hg
Ag
Au
Pt
as per according to question:
a) NaCl with copper , here Na is upper as compared to Cu so it will not replace element. copper is less reactive.
b) MgCl2 with aluminium, here Mg is upper as compared to Al, again replacement cannot occur, aluminium is less reactive than magnesium.
c) FeSO4 with silver, here Fe is upper as compared to Ag(silver), so replacement doesn't happen.
d)AgNO3 and Copper, here Ag is lower as compared to Cu(Copper), so replacement occur. This will produce a displacement reaction.2. Which of the following methods is suitable for preventing an iron frying pan from rusting?
(a) Applying grease
(b) Applying paint
(c) Applying a coating of zinc
(d) All of the above.
Ans: Option (c) Applying a coating of zinc
Explanation: Grease will not last long on the pan, applying paint is also not an ideal solution because the high heat used during cooking can cause the paint to become damaged or degrade over time, making it less effective at preventing rust.
So best will be applying zinc coating on the pan.3. An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be
(a) calcium
(b) carbon
(c) silicon
(d) iron.
Ans: Option a) Calcium
Explanation: The element that reacts with oxygen to give a compound with a high melting point and is also soluble in water is likely to be calcium (Ca), which forms calcium oxide (CaO) when it reacts with oxygen, and calcium hydroxide (Ca(OH)2) when it reacts with water. Calcium oxide has a high melting point, and calcium hydroxide is soluble in water.4. Food cans are coated with tin and not with zinc because
(a) zinc is costlier than tin.
(b) zinc has a higher melting point than tin.
(c) zinc is more reactive than tin.
(d) zinc is less reactive than tin.
Ans: Option c) Food cans are coated with tin and not with zinc because zinc is more reactive than tin.
Explanation: Zinc is more reactive than tin, and it would react with acidic foods, leading to the formation of undesirable compounds and potentially contaminating the food. Tin, on the other hand, is less reactive and more suitable for use as a protective coating for food cans.5. You are given a hammer, a battery, a bulb, wires and a switch.
(a) How could you use them to distinguish between samples of metals and non-metals?
(b) Assess the usefulness of these tests in distinguishing between metals and non-metals.
Ans: a) We can use these items to distinguish between samples of metals and non-metals.
We can use hammer, if any substance can form sheet after hammering it, then it is said to be a metal otherwise a non-metal. As metal have the property of malleability(ability to form sheets) and non-metals has property of brittleness(break or shatter into pieces ).
We can use Battery, bulb, wire and a switch to form a circuit, through which we can test, the substance is metal or not, metal conduct electricity and non-metal doesn't.
b)These tests are useful for a basic differentiation, but keep in mind that some materials, like sodium which is a metal but breaks when beaten with hammer and graphite which is an allotrope of the carbon(non-metal) but conducts electricity. 6. What are amphoteric oxides? Give two examples of amphoteric oxides.
Ans: All those metal oxides which reacts with both acid and base and form salts and water are called amphoteric oxides. For example Al2O3 and ZnO.
a)Al2O3 + 2NaOH -> 2NaAlO2 + H2O
Al2O3 + 6HCl -> 2AlCl3 + 3H2O
b)ZnO + 2NaOH -> Na2ZnO2 + H2O
ZnO + 2HCl -> ZnCl2 + H2O7. Name two metals which will displace hydrogen from dilute acids, and two metals which will not.
Ans: Na (sodium) and Ca (Calcium) can replace hydrogen from dilute acids, where as Cu (copper) and Ag(silver) will not replace hydrogen from dilute acids.
Explanations: All the metals which come before H(hydrogen) in reactivity series will replace the hydrogen and which come after hydrogen in series will not replace it. 8. In the electrolytic refining of a metal M, what would you take as the anode, the cathode and the electrolyte?
Ans: Anode: Thick plate of impure metal M
Cathode: Thick plate of pure metal M.
Electrolyte : An Aqueous solution of a salt of metal M.9. Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in figure below.
(a) What will be the action of gas on
(i) dry litmus paper?
(ii) moist litmus paper?
(b) Write a balanced chemical equation for the reaction taking place.
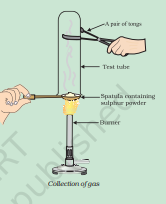
Ans: a) i)No action of SO2 gas on dry litmus paper
ii) SO2 Gas will turn moist blue litmus paper to red.
b) S(s) + O2(g)-> SO2(g)
SO2(g) + H2O(l) -> H2SO3(aq)
Explanation: When Sulphur is heated it will react with oxygen and form Sulphur oxide SO2. When it reacts with moisture means water particles it will form acid H2SO3 which turns blue litmus red.10. State two ways to prevent the rusting of iron.
Ans: The rusting of iron can be prevented by painting and galvanizing.
Painting: Applying a coat of paint to the iron surface creates a barrier that prevents oxygen and moisture from coming into direct contact with the metal.
Galvanization is a method of protecting steel and iron from rusting
by coating them with a thin layer of zinc.11. What type of oxides are formed when non-metals combine with oxygen?
Ans: When non- metals combine with oxygen it forms acidic oxide or neutral oxides. For example: H2O, CO, CO2, NO, SO2, P4O10
Among these H2O, CO, NO are neutral oxides and remaining are acidic oxides.12. Give reasons
(a) Platinum, gold and silver are used to make jewellery.
(b) Sodium, potassium and lithium are stored under oil.
(c) Aluminium is a highly reactive metal, yet it is used to make utensils for cooking.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
Ans: a) because they have properties like malleability, ductility, lustrous and do not corrode due to less reactivity.
b)because they are highly reactive, while reacting with oxygen they can cause fire due to exothermic reactions and they also react with water to form base.
c)Aluminium forms a protective layer of oxide because of that layer it does not react with food at high temperature and prevent from corrosion also.
d)Because it is easy to extract carbonate and sulphide from the oxides.13. You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Ans: Copper vessels gets tarnished due to formation of copper carbonate layer on the surface of vessel. Copper carbonate is basic in nature, so by applying lemon juice and tamarind which contains citric acid & tartaric acid respectively forms a water soluble solution. When these acid reacts with base it forms copper citrate and copper tartrate which are washed away with water. Hence vessel shines again.14. Differentiate between metal and non-metal on the basis of their chemical properties.
Ans: Metals:
1. Metals are electropositive and form cations.
2. Metals are reducing agents.
3. Metals from basic and amphoteric oxides.
4. Metals displace Hydrogen from dilute acids.
5. Metals react with chlorine to form solid ionic chlorides.
Non Metals:
1. Non-Metals are electronegative and form anions.
2. Non-Metals are oxidising agents.
3. Non-Metals form acidic and neutral oxides.
4. Non-Metals do not react with dilute acids and hence do not replace hydrogen.
5. Non-Metals react with chlorine to form volatile, covalent and liquid chlorides.15. A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Ans: The solution used to reduce the gold was Aqua Regia. It is a mixture of concentrated HCl (hydrochloric acid) and HNO3 (Nitric acid) in the ratio of 3:1 by volume. It dissolves the gold which reduce the weight of gold bangles.16. Give reasons why copper is used to make hot water tanks and not steel (an alloy of iron).
Ans: Copper is used to make hot water tanks instead of steel because steel is an alloy of iron. Iron reacts with oxygen and moisture to form rust. Where as copper forms a protective oxide layer due to which it does not corrode.